New Approaches in Cancer Immunotherapy
Chimeric antigen receptor T cells: New Approaches in Cancer Immunotherapy
Cancer is the second most common cause of death worldwide after cardiac arrest. Radiotherapy, surgery, and chemotherapy are some of the therapies used in cancer treatment. However, the problem associated with these therapies is that they have serious side effects over the long run on the human body and also, and the chances of recurrence are very high. Immunotherapy is the immerging and widely used therapy for cancer. Recent studies have shown that immunotherapy is beneficial against several cancers ranging from myelomas to lymphoma.
In immunotherapy, immune cells from a person are modified to attack and eradicate an antigen or cancer cell. The essential function of the immune system in eliminating malignant cells, particularly the function of T cells, is supported and demonstrated by an abundance of data. In the past few decades controlling and harnessing the immune system for practical clinical efficacy has been the mainstay of many immunotherapy therapies. Numerous immunotherapies that make use of oncolytic viruses, monoclonal antibodies and immune checkpoint inhibitors antibodies have undergone more research. Adoptive cell therapies involving Natural Killer cell therapy, Tumor-Infiltrating Lymphocytes therapy, T cell receptor engineered T cell and Chimeric Antigen Receptor T cell therapy has been enormously studied. In the current article, we are shedding some light on the structure of CAR T cell and its applications in Cancer. Chimeric Antigen Receptor T cell (CAR T cells) are the T lymphocytes that are genetically engineered to produce artificial T cell receptors for immunotherapy.
Chimeric Antigen Receptor (CARs) are the natural receptors that provide unique properties to T cells for attacking specific targeting proteins/antigens or cancerous cells. CAR T cell therapy has emerged as a novel therapeutic technique that now appears as a revolutionary therapeutic approach in cancer treatment. The significant benefit of this therapy is that tumour antigen is directly identified without the need for a major histocompatibility complex for antigen expression. Gross and Associates are recognized as a pioneer of CAR T cell therapy in cancer treatment. They reported the role of genetically modified cytotoxic T lymphocytes in tumor cells and concluded with the statement that “chimeric T cell receptors with antitumor specificity will enable testing the feasibility of this approach in combating human tumors”.
Detailed Structure of Chimeric Antigen Receptor
The structure of CARs is typically made up of four domains or regions: the hinge region, the antigen recognition domain (known as the ectodomain), the intracellular T cell signalling domain or the endodomain, atransmembrane domain as depicted in Figure 1.
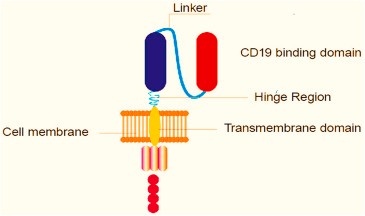
Fig 1: Diagrammatic representation of Chimeric Antigen Receptor structure
Antigen Recognition Region
The antigen recognition site detects antigens in the receptor's ectoderm. The target antigen interacts with this region, which is constantly exposed to the outside of the cell. The antigen recognition domain is derived from the variable region of monoclonal antibodies. Mostly, a single chain of variable fragments connects the domain (ScFv). ScFv is an immunoglobin chimeric protein that has both light (VL) and heavy (VH) chains. Short serine-glycine or glutamate-lysine linker peptides or glutamate-lysine linker peptides are linked with light and heavy chains.
Hinge region
It typically lies between the T cell's outer membrane and antigen recognition domain, alternatively, it is also known as a spacer. The increased accessibility of CAR-T cells to the antigen as a result of spacers gave receptors more flexibility. Studies have demonstrated, the antigen's epitope's location affects the optimum length of the spacer. Long spacers are frequently employed for membrane-proximal epitopes because they make CARs more flexible, which allows them to connect to complex glycosylated antigens' membrane-proximal epitopes more successfully.
Short spacer CARs can easily be attached to an antigen's distal membrane epitope. The performance of CAR T cells as a whole is significantly influenced by hinges or spacers. IGg derived spacer has two domains CH2 and CH3. Numerous studies have reported that IGg-based spacer incorporation into CARs frequently binds to the Fc gamma receptor (FcR) with the aid of the CH2 domain. This characteristic feature enables myeloid and lymphoid cells to express FcR to activate off-target and is associated with poor engraftment and off-tumor localization in animal models.
Transmembrane Domain of CAR
CARs transmembrane domain is located in between the intracellular signaling domain and the Hinge domain. The transmembrane domain is mainly derived from CD3-ζ, CD4, CD8, and sometimes from CD28 molecules. The transmembrane domains between the ectodomain and endodomain of the CARs were previously thought to be passive or non-reacting structural connections. However, it has been demonstrated through numerous tests that the transmembrane domain is crucial to the effects of CAR-T cell effector function. Hydrophobic alpha-helix structure stabilises the entire chimeric antigen receptor.
Intracellular T-cell SignalingDomain
In the Endodomain of the CAR, intracellular T cell signaling domain is present. When an antigen binds firmly to the antigen recognition region i.e extracellular domain, CARS receptors, in turn, come closer and simultaneously form a cluster which eventually transmits an activation signal to the inside of the cell. CD-3 Similar to other T cells, uses its cytoplasmic domain as the primary intracellular signalling domain (Endodomain), and CAR T cell activation requires a co-stimulatory domain or substances. Some co-stimulatory molecules that have been employed and studied as signalling domains include CD-27, CD-28, CD-134, and CD-137.
Manufacturing of CAR-T Cell
The manufacturing of CAR-T cell is based on six steps:
Lymphocyte’s isolation, T cell selection, Gene transfer, T cell expansion, formulation, and quality assurance or quality control.
Isolation of Lymphocytes
Prior to isolating lymphocytes during a Complete Blood Count test, the skin's needle administration site is cleaned with 2% chlorohexidine and 70% alcohol. 1% Lidocaine as anaesthesia is injected into the same area of the skin. The antecubital area of the "Inlet" arm and the "Receiving" arm both receive two arteriovenous fistula needles. Whole blood is pushed through an internal pump that helps maintain a constant flow rate of 2 ml/S of blood prior to entering the leukoreduction filter. The leukoreduction filter typically has a diameter of 8.7 cm and a blood volume holding capacity of 97 ml. It can process 1.5 L of blood at once. Leukocytes, particularly large lymphocytes, are trapped in the fibre mesh filter's pores but erythrocytes are allowed to pass through. Once the required amount of blood has been processed, the external and internal values have been closed, and the patient is then detached from the machine. 150 ml of a solution containing 3% 0.05 M EDTA and Phosphate Buffer Saline is used to extract leukocytes from the filter.
Density Gradients Centrifugation
Density gradients are a set of four-step centrifugation processes. Every stage of centrifugation is monitored by a particle dispersion analyser device. Leukocytes are removed from the EDTA/PBS solution at 250 g for 7 minutes in the initial stage. At the bottom, leukocytes settle and create a sturdy pallet. PBS/EDTA-containing supernatant is discarded. The pallet is resuspended and spun at 580 g for at least 15 minutes in the centrifuge. Three layers get separated and appear in the tube at the end of the process. Transfer the lymphocytes and monocytes to another tube and discard the other part of the tube. Again, both the monocytes and lymphocytes are resuspended in NaCl solution and centrifuged at 580 g for 15 minutes where monocytes form three layers of cells and lymphocytes get settled down in the centrifuged tube. Remove the monocyte and transfer the settled lymphocyte pallet with the help of a micropipette. Finally, the transferred cells are suspended in the DMSO solution in order to prepare the cells for cryopreservation.
Gene Transfer
There are typically two methods for transferring a gene into a T cell: viral genetransfer and non-viral gene transfer. Adenovirus, retrovirus, Adeno-associated virus, and lentivirus are among the viral vectors employed in the production of CART cells; retrovirus, which has undergone genetic engineering, is the most well-known and often utilized viral vector. Viral vectors are the most commonly used gene therapy because they have a high gene transfer efficiency rate and also, a variety of viruses with varying expression characteristics are readily available. Some non-viral vectors used for gene transfer in CAR T cell manufacturing procedures include naked DNA, liposomes, and a new plasmid base expression system known as the sleeping beauty transposons. These vectors are highly effective, have high target specificity, and have an infinite gene-carrying capacity.
T Cell Expansion
After the CAR construct has been retrovirally transduced, CAR T cells are grown for the following one to two weeks. In small-scale, static culture techniques like T flasks and wave bags, T cell growth is primarily accomplished. T cells do not develop in a highly agitated system because they are highly shear sensitive and need stable, static circumstances. T cell growth is facilitated by the stable and shear-free environments offered by T flasks and wave bags. Due to its high throughput capabilities, the ambr 250 Automate bioreactor is currently the most used bioreactor system for T cell growth. Due to automation and the presence of pumps, liquid handlers, and sensors, the risk of cross-contamination is decreased.
Formulation and Quality Check
The final T-cell product must pass stringent regulations andtoxicity testing developed by the Food and Drug Administration Authority before being administered into the patient's body (FDA). This phase is essential since any quality issue could force the FDA to conduct a thorough investigation and shut down the entire plant, costing time and money. The following major assays are frequently carried out for final quality assurance: FACS analysis for immunotyping; endotoxin detection using the Endo safe Portable Test System to determine the level of endotoxin in the final products; and chromium release assays to determine the level of cytotoxicity of T cells and, the Trypan blue exclusion method can be used to count viable cells (Figure 2).

Fig 2: CAR-T cell manufacturing steps
T Cells & Cancer
T cells are at the top of the immune surveillance system. Once the T cell receptor (TCR) binds to its cognate peptide present within the surface of the protein encoded by MHC (major histocompatibility complex) family of genes on an antigen-presenting cell. TCR binding is a more complex interaction than the relatively easy binding of a B cell antibody to an antigen due to the recognition of both an MHC and peptide. The T cell requires a second signal via co-stimulatory receptors once a TCR attaches to its associated peptide-MHC in order to fully activate
The subsequent release of cytokines by activated T cells starts a cycle of proliferation and differentiation. T cells can only respond to peptides that have been digested and are displayed on native MHCs, and they also require a second signal to fully activate. This process limits T cell responsiveness in a manner that B cell responsiveness does not. Viruses, bacteria, or proteins expressed without being processed or in a foreign MHC complex do not elicit any immunological response. The absence of a costimulatory signal results in T cell anergy.
The capability of T cells to identify foreign antigens and trigger an immune response has drawn researchers' interest to investigate whether T cells could recognise and eradicate tumor cells. Evasion is accomplished via numerous biological mechanisms. First, since T and B cells aren't exposed to any non-self-peptides or proteins, cancers may not trigger an immune response. However, several stress-related genes that activate a T cell response are upregulated by cancer cells. If such a reaction is ineffective in eliminating the cancerous cells, the remaining cells may subsequently halt the proteolytic process that produces peptides for display in MHC proteins or downregulate the expression of MHC proteins on the cell surface in order to avoid immune detection. Cancer cells modified to display a foreign peptide or protein on their cell surfaces have been used in mouse models to show that the immune system may effectively eliminate cancer cells if it can recognise them as non-self. Taking this into account, researchers started looking at alternative strategies for inducing an immunological response in people to cancer.
Advancements in CAR-T Cells
With the success of CAR-T cell therapies, researchers are still working to improve the technology in order to reduce or eliminate the manufacturing process and enhance the lot number of patients that can be treated per batch. One area of focus is the advancement of allogeneic CAR-T cell therapies through the clinical pipeline. Since several individuals can be treated with CAR-T cells, allogeneic therapies are more economical because the price per dosage is lower. Healthy donor CD34+ hematopoietic stem cells (HSCs) are transformed using plasmid technology to create the so-called Sleeping Beauty-engineered CAR-T cells. Identification of ultra-specific T cell phenotypes to increase transformation efficacy and shorten manufacturing timelines is another CAR-T discovery. In order to shift CAR-T cell proliferation back within the patient's own body after injection, Novartis' T-ChargeTM CAR-T platform selectively protects the naive and stem cell memory T cell (Tscm) populations. This reduces the manufacturing time to 24 hours from several weeks. Other technological advancements go even further to completely stop the ex-vivo manipulation of T cells. Adeno-associated virus (AAV) with a CAR gene can be delivered into the in-vivo environment to convert T cells into CAR-T cells, according to mouse research. Although it is still in the early phases of development, this approach has the potential to change the way that viral vector manufacture is approached in CAR-T research.
`Engineering T cells to boost the immune system has potential benefits beyond cancer treatment, including treatments for HIV/AIDS, chronic inflammatory disease, arthritis, and lupus. CAR T-cell therapy in organ transplantation is under investigation to eliminate the requirement for lifelong immunosuppressants. The future is bright for CAR-T research, and BPS Bioscience continues to develop unique cell lines and other tools to help researchers create, evaluate, and enhance CAR-T cells for the improvement of human health.
The future of CAR-T research is promising, and BPS Bioscience is working hard to establish novel cell lines and other resources to aid in the development, testing, and enhancement of CAR-T cells for the advancement of human health (https://bpsbioscience.com). BPS has developed stable, recombinant cell lines expressing cancer antigens at different expression levels--high, medium, or low expression. These cell lines are ideal to monitor the effectiveness of different CAR-T at detecting the target antigen expressed at different levels, which may relate to the time dependence of antigen expression.
BPS also offers biotin-labeled versions of many of the antigens recognized by CAR-T cells, such as BCMA, CD19, CD22, CD123, CD38, and ROR1. These biotin-labeled proteins can bind to CAR-T cells and be detected by flow cytometry using PE-streptavidin, in order to verify that the CAR-T cell is recognizing and binding the specific cancer antigen. BTL Biotechno Labs Pvt. Ltd. is the distributor of BPS Bioscience in India, supplying the CAR-T cell therapy products to scientific community. To explore the wide variety of life science products, follow the product section link https://biotechnolabs.com/products.
For product details, please connect with us at info@biotechnolabs.com.