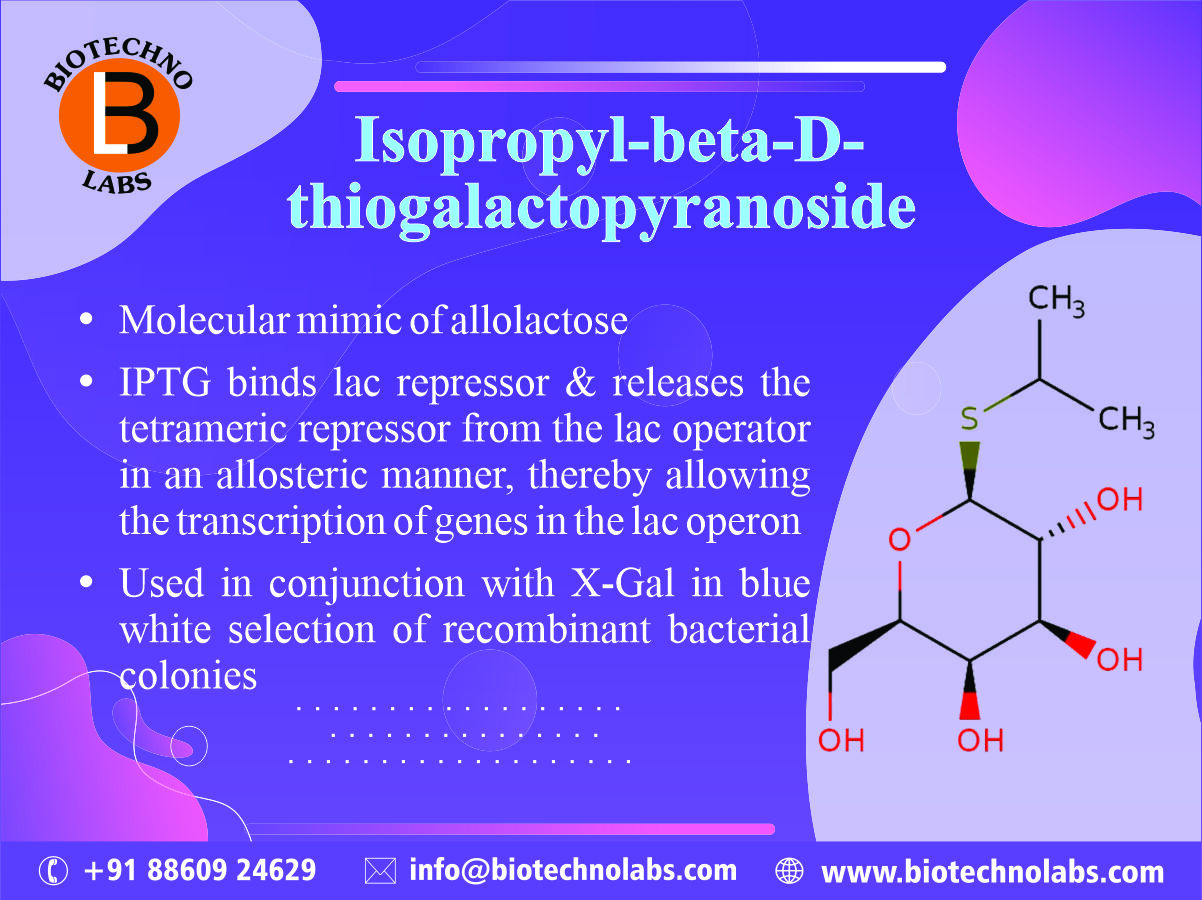
Lentivirus Biology: Insights, Innovations, and Applications
Molecular biology serves as the basis for various biotherapeutics, including recombinant
enzymes (e.g., factor IX monoclonal antibodies (e.g., trastuzumab), and growth factors (e.g.,
erythropoietin). Gene therapy, involving the administration of DNA encoding a desired gene
into cells to treat diseases, extends the reach of molecular biology to potentially alter the
genetic deformities (e.g., β-thalassemia due to β-globin gene defects). Notable examples
include adoptive cellular therapy using genetically engineered T cells, which, via synthetic
genes like chimeric antigen receptors (CARs) or cloned T-cell receptors (TCRs), gain the
ability to target antigens not recognized by their native TCRs. This approach has shown
promising clinical responses, even in patients with high B-cell malignancies.
Gene therapy via lentiviruses, gammaretroviruses, adenoviruses, and adeno-associated
viruses has gained attraction due to viruses' innate ability to transferr genetic material into
cells. Gammaretroviruses and lentiviruses, both retroviral subtypes with an RNA genome
converted to DNA by the viral enzyme reverse transcriptase in transduced cells, are
particularly noteworthy. While gammaretroviral vectors are more commonly used, especially
in research, the number of clinical trials useing lentiviral vectors is increasing.
Lentivirus Genome
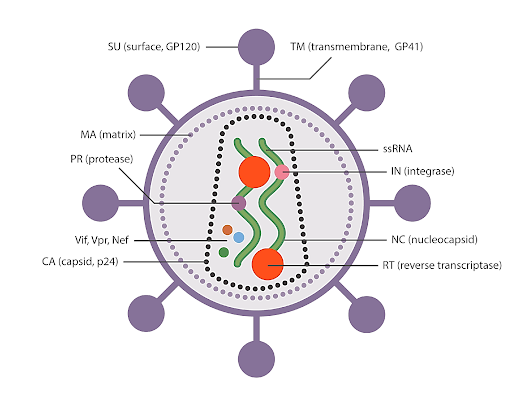
Gag, pol, and env are the essential genes for the survival and function of retroviruses and
lentiviruses. Gag encodes structural proteins; pol encodes enzymes necessary for reverse
transcription and integration into the host cell genome; and env encodes the viral envelope
glycoprotein. Retroviruses share a similar life cycle, beginning when the mature virus enters
the cell either through direct membrane fusion or receptor-mediated endocytosis, facilitated
by the binding of envelope glycoproteins to receptors on the cell's surface. Following this
fusion, the virus undergoes uncoating, during which several viral proteins, including some
Gag subunits, dissociate from the viral core. The viral RNA is then converted into proviral
double-stranded DNA through a complex process of reverse transcription. Subsequently, the
proviral DNA complexes with viral proteins to facilitate nuclear import and integration into
the host genome, a process assisted by crucial viral proteins like integrase and host cell
transcription factors such as LEDGF.
In the case of wild-type lentivirus, the integrated proviral genome relies on host machinery to
initiate and complete the transcription and translation of viral proteins necessary for
assembling infectious particles. These viral progenies exit the cell through a process called
budding, wherein virions are released into the extracellular space from the plasma membrane,
distinct from the budding process of other viruses. Lentiviruses, like many enveloped viruses,
utilize the endosomal sorting complexes required for the transport pathway to execute this
complex budding process and release virions into the extracellular space. During budding,
endogenous membrane proteins present within the host cell, such as MHC molecules, can be
incorporated into the virion envelope, potentially influencing the disposition of liberated viral
particles.
The retroviral life cycle involves two unique steps, reverse transcription and integration,
which are fundamental to the functioning of lentiviral vectors. After uncoating, the remaining
viral nucleic acid and protein complex form, which is known as the reverse transcription
complex (RTC). This RTC undergoes a series of steps to convert the single-stranded RNA
genome of the virus into double-stranded DNA.
Reverse transcription begins with a transfer of RNA binding to the primer-binding site at the
5' end of the viral RNA genome. As the negative strand of viral DNA is synthesized by the
polymerase activity of the reverse transcriptase enzyme, the viral RNA is degraded by the
RNase H activity of the same enzyme. This process develops a short fragment of negative-
sense, single-strand DNA, termed strong-stop copy DNA (sscDNA), which then serves as a
primer for the synthesis of the negative-strand viral DNA.
Multiple priming steps lead to the synthesis of a complete DNA copy of the viral RNA, with
an identical U3RU5 sequence within the long terminal repeat (LTR) at both ends. As reverse
transcription progresses, the complex transitions into a preintegration complex (PIC). During
this process, flaps of overlapping positive-strand DNA may form, potentially repaired by host
DNA repair enzymes post-integration. While the central polypurine tracts (or the flaps of
DNA they generate) are not essential for reverse transcription, their presence appears to
enhance the potency of viral vectors, possibly by accelerating second-strand synthesis and
protecting the RTC from innate restriction factors like APOBEC. Additionally, it's suggested
that the three-stranded DNA flap may enhance nuclear import. However, whether reverse
transcription is completed in the cytoplasm or nucleus remains unclear.
The specific composition of the reverse transcription complex (RTC) may enhance the
efficiency of reverse transcription. The capsid seems to shield the viral nucleic acid from
innate sensors while also facilitating more accurate reverse transcription with fewer errors. In
cell lines, reverse transcription is more precise compared to cell-free systems with a slower
rate (70 nt/min vs. 1000 nt/min). This rate further decreases in primary cells like
macrophages and resting T cells due to lower nucleotide pools.
Integration involves several steps, including tethering, 3' processing/cleavage, strand transfer,
and DNA repair. Notably, differences between gamma retroviruses (e.g., murine leukaemia
virus [MLV]) and lentiviruses (e.g., human immunodeficiency virus [HIV]) have certain
implications for designing retroviral gene therapy vectors. Gamma retroviruses like MLV
display a preference for insertion near transcriptional start sites, while lentiviruses like HIV
favor insertion within transcriptional units. Lentiviruses, with their ability to cross intact
nuclear pores, have additional integration criteria related to this unique capability, unlike
gamma retroviruses, which rely on nuclear envelope disassembly during mitosis.
Furthermore, host proteins within the nuclear pore, such as Nup153, Nup98, Nup358, CPSF6,
and TPR, are implicated in facilitating HIV's import. Studies suggest that the capsid protein
of HIV, but not MLV, enables HIV's translocation across the nuclear pore. In non-dividing
cells, integration site selection is influenced by proximity to the nuclear pore, with
heterochromatin typically associated with the nuclear envelope and actively transcribed genes
closer to the pore.
Host proteins LEDGF, BAF, and HMG are all associated with the pre-integration complex
(PIC) of HIV-1. LEDGF interacts with the PIC and epigenetic marks (H3K36me3),
facilitating integration within transcription units. These distinctive characteristics of
lentiviruses could be advantageous for gene therapy platforms targeting quiescent cell types
like long-term hematopoietic stem cells (HSCs).
Traditionally, it was believed that HIV couldn't infect quiescent CD4+ T cells in the G0 stage
but could infect activated CD4+ T cells. However, studies showed HIV DNA in quiescent
CD4+ T cells, suggesting it might originate from previously infected activated T cells
returning to a resting state. Although quiescent CD4+ T cells express HIV fusion receptors,
they exhibit resistance to infection due to factors like limited nucleotides and high expression
of restriction factors, slowing down reverse transcription. Despite this, integrated proviruses
can accumulate in resting CD4+ T cells over a longer period of time compared to activated T
cells.
Another challenge for lentivector infection of resting cells is the inefficient fusion of
quiescent CD4+ T cells with the commonly used viral envelope protein, vesicular stomatitis
virus glycoprotein (VSV-G). VSV-G receptor-mediated endocytosis isn't efficient in resting
CD4+ T cells, but treatment with certain cytokines like interleukin 7 can overcome this
limitation and enhance cell survival.
Lentiviral vectors
The first-generation lentiviral vectors comprised a substantial portion of the HIV genome,
encompassing the gag and pol genes, along with various additional viral proteins. To
facilitate cell entry, these vectors incorporated the envelope protein from another virus,
typically VSV-G, recognizing a widely expressed receptor identified as the low-density
lipoprotein (LDL) receptor. The VSV-G gene was housed separately from the lentiviral
genes. First-generation vectors included accessory and regulatory genes like vif, vpr, vpu,
nef, tat, and rev, which conferred survival benefits for viral replication in vivo but were
dispensable for in vitro growth. Safer, second-generation vectors were subsequently
developed, lacking these accessory virulence factors.
Further safety improvements were made with third-generation lentiviral vectors, which
fragmented the viral genome into separate plasmids to reduce the likelihood of recombinant
virus generation. In this system, the gag and pol genes were on a separate plasmid from the
rev or env genes, resulting in a vector assembled from three separate plasmids containing the
necessary viral sequences. The tat gene was replaced in third-generation vectors by a
constitutively active promoter in the upstream LTRs of the transgene construct. Self-
inactivating (SIN) lentiviral vectors, created by introducing deletions into the 3'LTR of the
viral genome, further enhance safety by disrupting LTR promoter/enhancer activity.
The choice of internal promoters in third-generation SIN lentiviral vectors is important, with
variations observed in their activity across cell types. Although most gene therapy approaches
activate T cells to divide before transduction, lentiviral vectors possess hypothetical
advantages in transducing non-dividing T cells, potentially retaining functional potential.
This targeting strategy may also reduce oncogenic potential compared to vectors targeting
actively dividing cells.
Clinical data indicate that new generation lentiviral vectors significantly reduce the risks of
insertional mutagenesis, with no reported cases of leukemogenesis in gene therapy trials
involving genetic modification of either HSCs or non-dividing T cells. While expansions of
cells with a common integration site have been observed, they do not appear to be related to
oncogenic selection. Lentiviral vectors have shown a lower risk of insertional oncogenesis
compared to gammaretroviral vectors in model systems, with SIN lentiviral vectors
demonstrating diminished genotoxic potential in clinically relevant mouse models.
Clinical Applications and Challenges of Lentivirus Vectors
Lentiviral and retroviral vectors are important technologies undergoing development for
various clinical applications requiring genetic material transfer. Lentiviral vectors have
gained prominence due to their efficiency in transducing non-proliferating or slowly
proliferating cells, such as CD34+ stem cells. The initial clinical use of lentiviral vectors
involved a conditionally replication-competent vector encoding an antisense RNA targeting
the HIV envelope gene, utilized for transducing mature peripheral blood T cells to treat
natural HIV infection. Integration site analysis demonstrated the expected preferential
integration within transcribed genes, with no significant alteration in integration site
distribution between pre-infusion cellular products and engrafted T cells. Lentiviral vector-
based gene transfer into CD34+ HSCs has since been applied in treating various genetic
diseases without adverse events reported in these trials. Notably, in a study transducing HSCs
with β-globin in β-thalassemia patients, one patient achieved independence from transfusion,
associated with an increase in a dominant myeloid clone bearing a lentiviral vector insertion
within the HMGA2 gene locus. The significance of this insertion within the dominant clone
remains uncertain.
Advances in cancer immunotherapy using genetically modified T cells, particularly CAR T-
cell therapies, have shown promising clinical responses in patients with B-cell malignancies.
Clinical trials utilizing CD19-targeted CAR T-cell therapy have demonstrated high efficacy
rates, with some safety concerns noted, and are primarily mechanism-based rather than
vector-related.
One notable on-target side effect of CD19 CAR T-cell therapy is B-cell aplasia, associated
with long-term CAR T-cell persistence. Lentiviral vector-modified T cells have shown
capability in persisting and inducing continued B-cell aplasia for extended periods following
treatment. Additionally, individuals receiving lentiviral vector-based gene therapies may yield
positive results on certain HIV testing platforms, necessitating careful differentiation from
natural HIV-1 infection.
Apart from ex vivo cell modification, lentiviral vectors are also being explored for direct in
vivo therapeutic applications. Clinical studies utilizing non-primate lentiviral vectors for local
gene delivery into the central nervous system and the eye have shown safety and potential
clinical benefit. However, challenges such as efficiency, tissue-restricted promoters, and
immunogenicity need to be addressed for the successful in vivo application of lentiviral
vectors. Surface engineering and genome editing of packaging cells hold promise for
enhancing the stability and efficacy of lentiviral vectors for in vivo use.
Ongoing research endeavors aim to enhance gene therapy efficacy through innovative viral
vector designs. Non-integrating lentiviral vectors (NILVs) have emerged as a promising
strategy to circumvent insertional mutagenesis risks. NILVs lack the viral integrase protein,
enabling transduction of both dividing and non-dividing cells while maintaining the viral
genome as an episome rather than integrating into the host genomic DNA. While the non-
integrating nature of NILVs may result in short-lived gene expression in dividing cells, this
transient expression could be advantageous in certain contexts, such as CAR T cell therapy,
where prolonged expression may not be required.
NILVs have demonstrated effectiveness as a vaccination approach in preclinical models,
eliciting cellular and humoral immunity along with anti-tumor responses. Additionally,
NILVs can be co-transduced with zinc finger nucleases to facilitate DNA recombination with
specific sites in the host DNA. Preclinical studies have shown the potential of zinc finger
nucleases to replace the endogenous T cell receptor (TCR) with a tumor-specific TCR
delivered by NILVs, thereby enhancing safety by mitigating off-target activity.
To enable long-term expression in dividing cells, a dual NILV vector system incorporating
the integrase of phage phiC31 has been developed. Lentiviral vectors have also been explored
in cancer vaccine development, including dendritic cell and cancer cell vaccines. These
vectors have been utilized to express tumor antigens in dendritic cells or modify
costimulatory signals to enhance efficacy. Additionally, lentiviral vectors have been
employed to activate the MAP kinase pathway in dendritic cells, resulting in enhanced anti-
tumor responses in murine models.
Another vaccine strategy involves using cancer cells expressing tumor antigens as the
vaccine. Lentiviral transduction of B-cell lymphoma cells with co-stimulatory proteins and
interleukin-12 demonstrated enhanced immunogenicity in murine models. Lentiviral vectors
have also been used to convert erythroleukemic cell lines into artificial antigen-presenting
cells for T cell expansion and potential in vivo vaccination.
Furthermore, lentiviral vectors have been utilized to deliver components of gene editing
systems, such as guide RNAs for the CRISPR-Cas9 system. These vectors enable precise
gene editing in target cells, offering potential alternatives to genetic screening methods.
Concerns about off-target effects have prompted the exploration of approaches to limit the
duration of gene editing, including the co-expression of guide RNAs targeting the Cas9
nuclease itself for degradation. Despite the early stage of development, lentiviral vectors
present a versatile platform for delivering gene editing tools and altering gene expression,
offering potential avenues for novel therapeutic interventions.
BTL Biotechno Labs Pvt. Ltd., an authorized distributor of LipExoGen in India, takes pride
in offering their comprehensive range of lentivirus, including TF reporter lentivirus, ORF
cDNA lentivirus, miRNA lentivirus, validated shRNA lentivirus, lentiviral particles, non-
lentiviral ORF cDNA plasmid, lentiviral ORF cDNA plasmid, and all plasmids, to the
scientific community for the advancement of research in drug discovery and gene therapy.
For product details, please connect with us at info@biotechnolabs.com.