Nitrogen Metabolism Antibodies in Plants
Nitrogen Metabolism is one of the most essential macro-elements that regulates plant growth, especially in agricultural fields, along with carbon, hydrogen, and oxygen. The atmosphere serves as the primary source of nitrogen for the development of nitrogenous organic molecules. It can be found in numerous active biomolecules, including proteins, phytohormones, nucleic acids, and several vitamins. Therefore, the majority of the biochemical reactions in living activities contain nitrogen, which is a component of these biomolecules as well as several other essential chemicals.
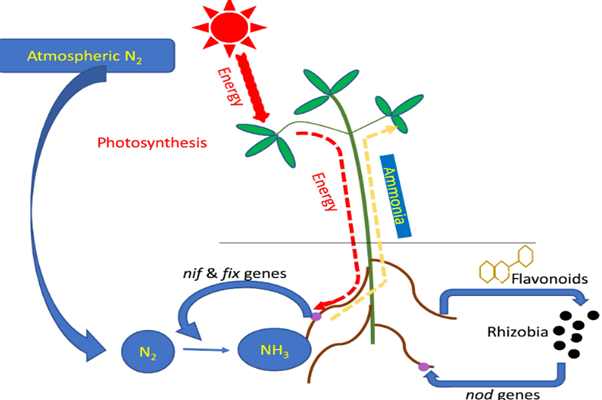
Nitrogen Metabolism is essential to plants' metabolism and structure. About 80% of the earth's atmosphere is composed of nitrogen, but most plants are unable to use it in its purest form. They must rely on the soil for its supply and obtain nitrogen in its inorganic form as nitrate or ammonium molecules. Only a few prokaryotic organisms may directly utilise air nitrogen. A few prokaryotic life forms in a stable ecological system transform atmospheric nitrogen into a metabolically useful form that can be supplied to higher plants and animals.
Ammonia, the most usable form of nitrogen, is first transformed into glutamate and glutamine, which are then further transformed into other nitrogen-containing molecules necessary for plant development and maintenance. Nitrate (NO3), ammonium ions (NH4+), or organic nitrogen, are derived through the nitrogen cycle, and farm manure is an additional source of nitrogen in the soil. Prior to metabolism, nitrate is transformed into ammonia. Plant roots can directly absorb ammonium ions and organic nitrogen in the form of amino acids liberated from proteins or partially degraded proteins.
With the decay of organic systems of the plant amino acids, purines and pyrimidines are cycled back to ammonia during the process some part of ammonia is lost from the N2 cycle which converts into NO2 – and to N2 gas or to NO3 –. Thus, anabolic and catabolic processes are both parts of nitrogen metabolism.
The anabolic processes are: a) nitrogen fixation; b) amino acid synthesis; c) protein synthesis.
The catabolic processes are: a) proteolysis and amino acid destruction. b) de-nitrification; c) nitrification.
It is therefore evident that nitrogen is available in several forms in the environment for the plant system, such as molecular nitrogen, organic nitrogen, ammonia, and nitrate. The continuous interconversion of these nitrogen forms by physical and biological processes is required to maintain the constancy of the amount of nitrogen in the atmosphere.
Steps of Nitrogen Metabolism cycle:
Three steps of Nitrogen cycle
- Ammonification and nitrification
- De-nitrification
- Nitrogen fixation
Nitrogen Metabolism Antibodies supplier in India
Ammonification and Nitrification:
Ammonification is the process by which soil bacteria convert organic nitrogen to NH3. Animal waste and decomposing plant and animal leftovers both contribute to the organic nitrogen in the soil. Ammonia is oxidised to produce nitrite and eventually nitrate in warm (30°–35°C) and moist soils with a 4pH. The process of oxidation is called nitrification and is carried out by microorganisms.
Bacillus mycoides, B. vulgaris, and B. ramosus are the ammonifying saprophytic bacteria. Certain actinomycetes and fungi have the ability to release ammonia from a natural organic compound. Nitrification is a process carried out by two chemoautotrophic bacteria known as Nitrosomonas and Nitrobacter
Nitrosomonas convert ammonia to nitrite and to nitrate by Nitrobacter as follows:
The nitrate reductases have been isolated from bacterial species like E. coli, Pseudomonas aeruginosa, Neurospora, and soybean plants. The enzyme forms homodimers and is a complicated metalloenzyme. It has nitrate and NAD(P)H binding sites.
The redox centres, formed by the cofactors FAD, heme-Fe, and molybdenum cofactor (MoCo), facilitate the electron transfer reactions:
MoCo is a molybdenum ion complex associated with the organic molecule pterin, a metal chelator. Each nitrogen reductase subunit is 1000 amino acids long, along with three cofactors. Most of the plant nitrogen reductases use NADH, while some plants use NADH or NADPH. The genes for nitrate reductase from several higher plants have been cloned.
The process is repeated when reducing nitrite is oxidised to NH3 through intermediates like hyponitrite and hydroxylamine. Each step involves the addition of two electrons by reduced NAD+ (NADP+). Each step requires decreasing NAD+ (NADP+) to add two electrons. Nitrate assimilation is the process by which aerobic microorganisms and higher plants reduce NO3- to NH3 and incorporate it into cellular proteins.
Nitrite reductase (NiR) transfers electrons from ferredoxin to nitrite as follows:
NO2 + 6Fdred + 8H+ + 6e → NH4 + 6Fdox + 2H2O
Reduced ferredoxin (Fdred), which is produced in the chloroplast photosynthetic noncyclic electron transfer, is the source of electron. As in the case of non-photosynthetic tissues, nitrite reduction utilises Fdred in plastids. The enzyme Fd-NADP+ reductase lowers the ferredoxin level with NADPH production through the oxidative pentose phosphate pathway. The polypeptide that makes up the enzyme NiR has two prosthetic groups: an iron-sulfur core (Fe4S4) and a particular heme (siroheme).
The enzyme is a nuclear-encoded protein that is cleaved from the mature enzyme to form an N-terminal transit peptide. The transit peptide directs the precursor peptide to the plastids. The monomer NiR has a molecular mass between 60 and 70 kDa, functional domains, and cofactors that transport electrons from Fdrcd to nitrite. The two functional domains are bridged by a sulphur ligand. The sulphur ligand connects the two functional domains. Together with NR, NiR's transcription is regulated. Cells must have enough NiR to completely reduce the nitrite produced by NR.
(ii) De-Nitrification:
De-nitrification is the process by which nitrate and nitrite are transformed into ammonia, nitrogen gas, and nitrous oxide (N2O). The nitrogen cycle is the process that results in the release of gaseous nitrogen into the environment. The anaerobic bacteria like Pseudomonas denitrificans and Bacillus subtilis convert nitrates to ammonia and free nitrogen through a sequence of reactions. Thyobacillus denitrificans, Azotobacter, Clostridium, Micrococcus, etc., use NO3 as an electron acceptor dburing respiration to obtain the energy for survival.
Nitrogen Fixation:
Nitrogen Fixation: The process of converting di-nitrogen (N2O) into an organic form so as to make it available for plants is known as nitrogen fixation. The ancient practise of rotational field crop of leguminous and non- leguminous was based on the fact high and better yield.
Nitrogen Metabolism Antibodies at the best price in India
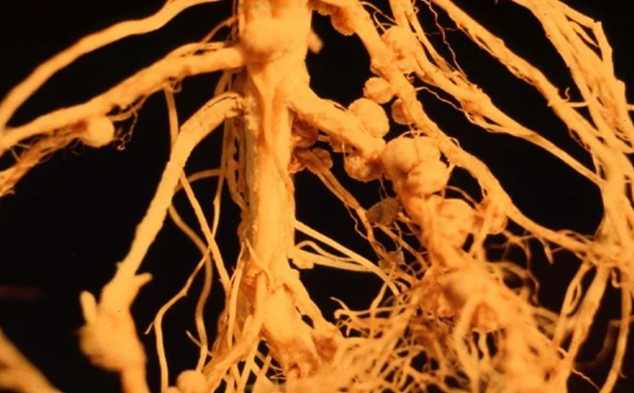
However, no one realised the advantages were related to atmospheric nitrogen fixation until it was established by Baussingault in 1838 that soil fertility was increased by the nitrogen-fixing bacteria found in the root nodules of leguminous crops.
Nitrogen fixation is of two types: non-biological fixation and biological fixation.
(a) Physical or non-biological nitrogen fixation
The nitrogen molecule is highly stable and exists as di-nitrogen (N2), which has a triple bond between two nitrogen atoms. The nitrogen bond is the shortest, with a length of 1.095, yet it also has the highest stretching frequency and ionisation potential (15.58 eV). It is therefore very resistant to chemical attack. About 225 kcal of energy are needed to break this triple bond, although it is exceedingly challenging to do so. Nitrogen is converted to ammonia in the fertiliser business at extremely high temperatures and pressures over an iron catalyst.
The electrical discharges that take place during lightning can also fix nitrogen. In this process, the nitrogen in the atmosphere reacts with the oxygen to form nitrogen oxides, which are then hydrated by water vapour and transported to earth as nitrites and nitrates by rain.
(b) Biological Nitrogen Fixation:
During the process of evolution, certain bacterial species have evolved the ability to fix and convert nitrogen to ammonia, a process that is controlled by a group of specific genes known as the nitrogen fixation (nlf) genes. They are referred to as "nitrogen-fixing" species. These microorganisms can either be non-symbiotic and exist on their own or coexist with higher plants. Rhodospirillum sp., photosynthetic bacteria (Azotobacter sp.), anaerobic bacteria (Clostridium sp.), and a number of blue-green algae are included in the former category (Cyanophyta).
The symbiotic system consists of several plant families like Leguminosae, including peas, beans, clovers, soybeans, and others, with bacteria from the genus Rhizobium to form a significant nitrogen-fixing cooperative. The growth of nodules on the plant roots is an essential feature of the symbiotic fixation. This article on nitrogen metabolism notes that includes multiple variants of this vital biological process for eg:- nitrogen fixation, assimilation, and the elimination of nitrogenous waste.
We, BTL Biotechno Labs Pvt. Ltd., along with our channel partner Agrisera, supply a collection of nitrogen metabolism antibodies targeting key enzymes of nitrogen metabolism in plants, reactivity ranges from algae to Arabidopsis thaliana.
Currently, we are offering a 20% discount on all nitrogen metabolism antibodies, valid from January to March 2023. We are an authorized Nitrogen Metabolism Antibodies supplier in India.
@Agrisera is a Swedish company established in 1985 that specializes in antibody production and purification. Agrisera provides off-the-shelf antibodies for plant and algae research, with prompt delivery worldwide and reactivity to thousands of species.
We are the authorised antibodies supplier of Agrisera in India. Contact our team to order Nitrogen Metabolism Antibodies at the best price in India.
For more details, please follow the below link:
https://biotechnolabs.com/products
Antibodies to plant enzymes involved in nitrogen metabolism (agrisera.com)
For product details, please connect with us at info@biotechnolabs.com.